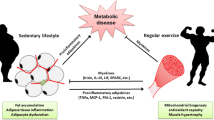
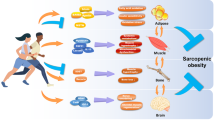
Abstract
Recent years have demonstrated clear evidence that skeletal muscle is an active endocrine organ. During contraction of muscle fibers, the skeletal muscle produces and releases, into the blood stream, cytokines and other peptides, called myokines, thanks to which it can both communicate with cells locally within the muscle, in an autocrine and paracrine fashion, or with other distant tissues, exerting its endocrine effects. With the progress of sophisticated technologies, the interest towards the skeletal muscle secretome is rapidly grown and the discovery of new myokines represents a prolific field for the identification of new pharmacological approaches for the management and treatment of many clinical diseases. Considering the importance of the muscle proteome and the cross-talk with other organs, the preservation of a skeletal muscle in good health represents a fundamental aspect in life, especially in ageing. Sarcopenia is the age-dependent loss of skeletal muscle mass and strength, bringing to increases of the risk of adverse outcomes, such as physical disability and poor quality of life, as well as alteration of several hormonal networks. For that reasons, the scientific community has risen its interest to find new interventions to prevent and manage the sarcopenia. Adequate nutrition during ages plays a fundamental role in the health and function of the skeletal muscle and it can represents, alone or in combination with physical exercise, a possible preventive measure against sarcopenia. This review will overview the endocrinology of the skeletal muscle, making a focus on food intake as a strategy for preventing skeletal muscle decay.


Similar content being viewed by others
References
Pedersen BK (2013) Muscle as a secretory organ. Compr Physiol 3:1337–1362. https://doi.org/10.1002/cphy.c120033
Pedersen I, Hojman P (2012) Muscle-to-organ cross talk mediated by myokines. Adipocyte 1:164–167. https://doi.org/10.4161/adip.20344
Marzetti E, Calvani R, Tosato M et al (2017) Sarcopenia: an overview. Aging Clin Exp Res 29:11–17. https://doi.org/10.1007/s40520-016-0704-5
Goldstein MS (1961) Humoral nature of the hypoglycemic factor of muscular work. Diabetes 10:232–234. https://doi.org/10.2337/diab.10.3.232
Ostrowski K, Rohde T, Zacho M et al (1998) Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508:949–953. https://doi.org/10.1111/j.1469-7793.1998.949bp.x
Steensberg A, van Hall G, Osada T et al (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242. https://doi.org/10.1111/j.1469-7793.2000.00237.x
Bruunsgaard H, Galbo H, Halkjaer-Kristensen J et al (1997) Exercise-induced increase in serum interleukin-6 in humans is related to muscle damage. J Physiol 499:833–841
Pedersen B, Steensberg A, Fischer C et al (2003) Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24:113–119
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
MacIntyre D, Sorichter S, Mair J et al (2001) Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol 84:180–186. https://doi.org/10.1007/s004210170002
Weigert C, Hennige A, Brodbeck K et al (2005) Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab 289:E251–E257. https://doi.org/10.1152/ajpendo.00448.2004
Glund S, Deshmukh A, Long Y et al (2007) Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes 56:1630–1637. https://doi.org/10.2337/db06-1733
Carey A, Steinberg G, Macaulay S et al (2006) Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55:2688–2697. https://doi.org/10.2337/db05-1404
Pedersen B, Akerström T, Nielsen A et al (2007) Role of myokines in exercise and metabolism. J Appl Physiol 103:1093–1098. https://doi.org/10.1152/japplphysiol.00080.2007
So B, Kim H, Kim J et al (2014) Exercise-induced myokines in health and metabolic diseases. Integr Med Res 3:172–179. https://doi.org/10.1016/j.imr.2014.09.007
Jy H (2018) The role of exercise-induced myokines in regulating metabolism. Arch Pharm Res 41:14–29. https://doi.org/10.1007/s12272-017-0994-y
X’avia Chan C, McDermott J, Siu K (2011) Secretome analysis of skeletal myogenesis using SILAC and shotgun proteomics. Int J Proteom 32:7. https://doi.org/10.1155/2011/329467
X’avia Chan C, Masui O, Krakovska O et al (2011) Identification of differentially regulated secretome components during skeletal myogenesis. Mol Cell Proteom 10:M110.004804. https://doi.org/10.1074/mcp.m110.004804
Yoon J, Kim J, Song P et al (2012) Secretomics for skeletal muscle cells: a discovery of novel regulators? Adv Biol Regul 52:340–350. https://doi.org/10.1016/j.jbior.2012.03.001
Ohlendieck K (2013) Proteomic identification of biomarkers of skeletal muscle disorders. Biomark Med 7:169–186. https://doi.org/10.2217/bmm.12.96
Brown K, Formolo C, Seol H et al (2012) Advances in the proteomic investigation of the cell secretome. Expert Rev Proteom 9:337–345. https://doi.org/10.1586/epr.12.21
Yoon J, Song P, Jang J et al (2009) Comparative proteomic analysis of the insulin-induced L6 myotube secretome. Proteomics 10:5315–5325. https://doi.org/10.1021/pr200573b
Hartwig S, Raschke S, Knebel B et al (1844) (2913) Secretome profiling of primary human skeletal muscle cells. Biochim Biophys Acta 5:1011–1017. https://doi.org/10.1016/j.bbapap.2013.08.004
Henningsen J, Rigbolt K, Blagoev B et al (2010) Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteom 9:2482–2496. https://doi.org/10.1074/mcp.M110.002113
Deshmukh A, Cox J, Jensen L et al (2015) Secretome analysis of lipid-induced insulin resistance in skeletal muscle cells by a combined experimental and bioinformatics workflow. J Proteome Res 14:4885–4895. https://doi.org/10.1021/acs.jproteome.5b00720
Grube L, Dellen R, Kruse F et al (2018) Mining the secretome of C2C12 muscle cells: data dependent experimental approach to analyze protein secretion using label-free quantification and peptide based analysis. J Proteome Res 17:879–890. https://doi.org/10.1021/acs.jproteome.7b00684
Raschke S, Eckardt K, Bjørklund Holven K et al (2013) Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One 8:e62008. https://doi.org/10.1371/journal.pone.0062008
Norheim F, Raastad T, Thiede B et al (2011) Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab 301:E1013–E1021. https://doi.org/10.1152/ajpendo.00326.2011
Pourteymour S, Eckardt K, Holen T et al (2017) Global mRNA sequencing of human skeletal muscle: search for novel exercise-regulated myokines. Mol Metab 6:352–365. https://doi.org/10.1016/j.molmet.2017.01.007
McPherron A, Lawler A, Lee S (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90. https://doi.org/10.1038/387083a0
Rodgers B, Garikipati D (2008) Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev 29:513–534. https://doi.org/10.1210/er.2008-0003
Hansen J, Brandt C, Nielsen A et al (2011) Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152:164–171. https://doi.org/10.1210/en.2010-0868
Pedersen B (2012) «Muscular interleukin-6 and its role as an energy sensor. Med Sci Sports Exerc 44:392–396. https://doi.org/10.1249/MSS.0b013e31822f94ac
Pedersen B, Febbraio M (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406. https://doi.org/10.1152/physrev.90100.2007
Quinn L, Anderson B, Strait-Bodey L et al (2009) Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab 296:E191–E202. https://doi.org/10.1152/ajpendo.90506.2008
Allen T, Whitham M, Febbraio M (2012) IL-6 muscles in on the gut and pancreas to enhance insulin secretion. Cell Metab 15:8–9. https://doi.org/10.1016/j.cmet.2011.12.004
Boström P, Wu J, Jedrychowski M et al (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468. https://doi.org/10.1038/nature10777
Jeremic N, Chaturvedi P, Tyagi S (2017) Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol 232:61–68. https://doi.org/10.1002/jcp.25450
Colaianni G, Cuscito C, Mongelli T et al (2014) Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014:902186. https://doi.org/10.1155/2014/902186
Colaianni G, Cuscito C, Mongelli T et al (2015) The myokine irisin increases cortical bone mass. Proc Natl Acad Sci USA 112:E5763. https://doi.org/10.1073/pnas
Hamrick M (2012) The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bonekey Rep 1:60. https://doi.org/10.1038/bonekey.2012.60
Wei K, Serpooshan V, Hurtado C et al (2015) Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525:479–485. https://doi.org/10.1038/nature15372
Ouchi N, Oshima Y, Ohashi K et al (2008) Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 283:32802–32811. https://doi.org/10.1074/jbc.M803440200
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94:12457–12461. https://doi.org/10.1073/pnas.94.23.12457
White TA, LeBrasseur NK (2014) Myostatin and sarcopenia: opportunities and challenges—a mini-review. Gerontology 60:289–293. https://doi.org/10.1159/000356740
Trendelenburg AU, Meyer A, Rohner D et al (2009) Myostatin reduces Akt/TORC1/p70S6 K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270. https://doi.org/10.1152/ajpcell.00105.2009
Schuelke M, Wagner KR, Stolz LE et al (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688. https://doi.org/10.1056/NEJMoa040933
Mosher DS, Quignon P, Bustamante CD et al (2007) A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet 3:e79. https://doi.org/10.1371/journal.pgen.0030079
Clop A, Marcq F, Takeda H et al (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38:813–818. https://doi.org/10.1038/ng1810
Saitoh M, Ishida J, Ebner N et al (2017) Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. JCSM Clin Rep 2:1e00037–10e00037. https://doi.org/10.17987/jcsm-cr.v2i1.37
Hoogaars WMH, Jaspers RT (2018) Past, present, and future perspective of targeting myostatin and related signaling pathways to counteract muscle atrophy (Chapter 8). In: Xiao J (ed) Muscle atrophy, advances in experimental medicine and biology, vol 1088. Springer, Singapore. https://doi.org/10.1007/978-981-13-1435-3_8
Reginster JY, Cooper C, Rizzoli R et al (2016) Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res 28:47–58. https://doi.org/10.1007/s40520-015-0517-y
Cruz-Jentoft A, Baeyens J, Bauer J et al (2010) European Working Group on Sarcopenia in Older People, Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Reginster J, Cooper C, Rizzoli R et al (2016) Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res 28:47–58. https://doi.org/10.1007/s40520-015-0517-y
Vitale G, Cesari M, Mari D (2016) Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med 35:10–15. https://doi.org/10.1016/j.ejim.2016.07.017
Landi F, Calvani R, Cesari M et al (2015) Sarcopenia as the biological substrate of physical frailty. Clin Geriatr Med 31:367–374. https://doi.org/10.1016/j.cger.2015.04.005
Calvani R, Miccheli A, Landi F et al (2013) Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging 2:38–53
Malafarina V, Uriz-Otano F, Gil-Guerrero L et al (2013) The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 74:293–302. https://doi.org/10.1016/j.maturitas.2013.01.016
Wolfe RR (2002) Regulation of muscle protein by amino acids. J Nutr 132:3219S–3224S. https://doi.org/10.1093/jn/131.10.3219S
Wolfe RR, Miller S, Miller K (2008) Optimal protein intake in the elderly. Clin Nutr 27:675–684. https://doi.org/10.1016/j.clnu.2008.06.008
Katsanos C, Kobayashi H, Sheffield-Moore M et al (2006) A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291:E381–E387. https://doi.org/10.1152/ajpendo.00488.2005
Katsanos C, Kobayashi H, Sheffield-Moore S et al (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82:1065–1073. https://doi.org/10.1093/ajcn/82.5.1065
Panel on Macronutrients, Panel on the Definition of Dietary Fibers, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board. Institute of Medicine of the National Academies (2005) Protein and amino acids. In: Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. The National Academies Press, Washington DC, pp. 589–768. https://doi.org/10.17226/10490
Morley J, Argiles J, Evans W et al (2010) Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 11:391–396. https://doi.org/10.1016/j.jamda.2010.04.014
Bauer J, Biolo G, Cederholm T et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14:542–559. https://doi.org/10.1016/j.jamda.2013.05.021
Symons T, Sheffield-Moore M et al (2009) A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 109:1582–1586. https://doi.org/10.1016/j.jada.2009.06.369
Kim I, Schutzler S, Schrader A et al (2015) Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab 308:E21–E28. https://doi.org/10.1152/ajpendo.00382.2014
Mitchell W, Wilkinson D, Phillips B et al (2016) Human skeletal muscle protein metabolism responses to amino acid nutrition. Adv Nutr 7:828S–838S. https://doi.org/10.3945/an.115.011650
Anthony J, Anthony T, Kimball S et al (2001) Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131:856S–860S. https://doi.org/10.1093/jn/131.3.856S
Anthony J, Yoshizawa F, Anthony T et al (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419. https://doi.org/10.1093/jn/130.10.2413
Nakashima K, Ishida A, Yamazaki M et al (2005) Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem Biophys Res Commun 336:660–666. https://doi.org/10.1016/j.bbrc.2005.08.138
Landi F, Calvani R, Tosato M et al (2016) Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients 8:295. https://doi.org/10.3390/nu8050295
Xu Z, Tan Z, Zhang Q, Gui Q, Yang Y (2014) The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr 113:25–34. https://doi.org/10.1017/S0007114514002475
van Vliet S, Burd N, van Loon L (2015) The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr 145:1981–1991. https://doi.org/10.3945/jn.114.204305
Yang Y, Churchward-Venne T, Burd N et al (2012) Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 9:57. https://doi.org/10.1186/1743-7075-9-57
Rondanelli M, Perna S, Faliva M et al (2015) Novel insights on intake of meat and prevention of sarcopenia: all reasons for an adequate consumption. Nutr Hosp 32:2136–2143. https://doi.org/10.3305/nh.2015.32.5.9638
Wu H, Xia Y, Jiang J et al (2015) Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 61:168–175. https://doi.org/10.1016/j.archger.2015.06.020
Robinson R, Reginster J, Rizzoli R et al (2018) Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr 37:1121–1132. https://doi.org/10.1016/j.clnu.2017.08.016
Pedersen BK (2009) The diseasome of physical inactivity–and the role of myokines in muscle–fat cross talk. J Physiol 587:5559–5568. https://doi.org/10.1113/jphysiol.2009.179515
Plomgaard P, Bouzakri K, Krogh-Madsen R et al (2005) Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54:2939–2945. https://doi.org/10.2337/diabetes.54.10.2939
Landi F, Marzetti E, Martone A et al (2014) Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care 17:25–31. https://doi.org/10.1097/MCO.0000000000000018
Marzetti E, Calvani R, Tosato M et al (2017) Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res 29:35–42. https://doi.org/10.1007/s40520-016-0705-4
Chesley A, MacDougall J, Tarnopolsky M et al (1992) Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 73:1383–1388. https://doi.org/10.1152/jappl.1992.73.4.1383
Beaudart C, Dawson A, Shaw S et al (2017) Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 28:1817–1833. https://doi.org/10.1007/s00198-017-3980-9
Deutz N, Bauer J, Barazzoni R et al (2014) Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Nutr 33:929–936. https://doi.org/10.1016/j.clnu.2014.04.007
Landi F, Cesari M, Calvani R et al (2017) The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res 29:89–100. https://doi.org/10.1007/s40520-016-0715-2
Marzetti E, Cesari M, Calvani R et al (2018) The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: case finding, screening and characteristics of eligible participants. Exp Gerontol 113:48–57. https://doi.org/10.1016/j.exger.2018.09.017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Romagnoli, C., Pampaloni, B. & Brandi, M.L. Muscle endocrinology and its relation with nutrition. Aging Clin Exp Res 31, 783–792 (2019). https://doi.org/10.1007/s40520-019-01188-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01188-5